Synthesis, characterization and molecular docking studies of acetamide derivatives of 2-aminothiazole and 2-dihydropyridinone derivative of benzimidazole
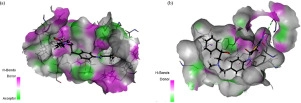
An efficient and easy synthesis of highly functionalized thiazole and pyridinone-substituted benzimidazole derivatives has been developed. The imino carbon of the benzimidazole derivative is coupled with the nitrogen atom of 1,4-dihydropyridinone and the aminothiazole-substituted acetic acid is condensed with two differently functionalized aniline, in two different reactions to form the amide linkages. These two amides and pyridinone-coupled benzimidazole are characterized by IR, 1H & 13C NMR and mass spectral studies. They are further evaluated for their antibacterial activity against the gram-positive bacterium Staphylococcus epidermidis and the fungus Saccharomyces cerevisiae by molecular docking method. All the three synthesized compounds had relatively lesser binding energy than what the standard drugs chloramphenicol and fluconazole have and may be considered as better inhibitors.
Publisher URL: https://www.sciencedirect.com/science/article/pii/S0022286021024352
DOI: 10.1016/j.molstruc.2021.132315
Keeping up-to-date with research can feel impossible, with papers being published faster than you'll ever be able to read them. That's where Researcher comes in: we're simplifying discovery and making important discussions happen. With over 19,000 sources, including peer-reviewed journals, preprints, blogs, universities, podcasts and Live events across 10 research areas, you'll never miss what's important to you. It's like social media, but better. Oh, and we should mention - it's free.
Researcher displays publicly available abstracts and doesn’t host any full article content. If the content is open access, we will direct clicks from the abstracts to the publisher website and display the PDF copy on our platform. Clicks to view the full text will be directed to the publisher website, where only users with subscriptions or access through their institution are able to view the full article.


