Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia
Importance
Agitation is a prevalent, distressing, and burdensome manifestation of Alzheimer dementia in need of an efficacious, safe, and well-tolerated treatment.
Objective
To confirm the efficacy, safety, and tolerability of brexpiprazole in patients with agitation in Alzheimer dementia.
Design, Setting, and Participants
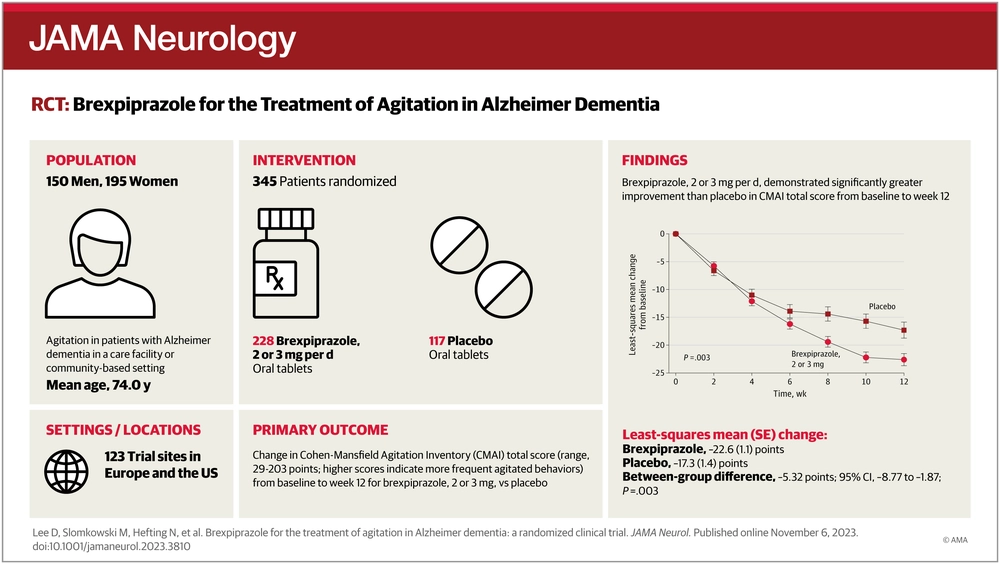
This randomized clinical trial was a 12-week, double-blind, placebo-controlled, fixed-dose, parallel-arm trial that ran from May 2018 to June 2022 at 123 clinical trial sites in Europe and the United States. Participants included patients with agitation in Alzheimer dementia in a care facility or community-based setting. Stable Alzheimer disease medications were permitted.
Interventions
In this 2-arm trial, patients were randomized to receive oral brexpiprazole or placebo (2:1 ratio) for 12 weeks. Within the brexpiprazole arm, patients were further randomized to receive fixed doses of 2 mg/d or 3 mg/d in a 1:2 ratio.
Main Outcomes and Measures
The primary end point was change in Cohen-Mansfield Agitation Inventory total score (which measures the frequency of 29 agitated behaviors) from baseline to week 12 for brexpiprazole, 2 or 3 mg, vs placebo. Safety was assessed by standard measures, including treatment-emergent adverse events.
Results
A total of 345 patients were randomized to receive brexpiprazole (n = 228) or placebo (n = 117); completion rates were 198 (86.8%) for brexpiprazole and 104 (88.9%) for placebo. Mean (SD) age was 74.0 (7.5) years, and 195 of 345 patients were female (56.5%). Patients receiving brexpiprazole, 2 or 3 mg (n = 225), demonstrated statistically significantly greater improvement than those taking placebo (n = 116) in Cohen-Mansfield Agitation Inventory total score from baseline to week 12 (brexpiprazole baseline, 80.6, mean change, −22.6; placebo baseline, 79.2, mean change, −17.3; least-squares mean difference, −5.32; 95% CI, −8.77 to −1.87;P = .003; Cohendeffect size, 0.35). No treatment-emergent adverse events had an incidence of 5% or more with brexpiprazole and greater incidence than placebo. The proportion of patients who discontinued because of adverse events was 12 of 226 (5.3%) for brexpiprazole and 5 of 116 (4.3%) for placebo.
Conclusions and Relevance
In this study, patients with Alzheimer dementia who took brexpiprazole, 2 or 3 mg, showed a statistically significant improvement vs placebo in agitation over 12 weeks. Brexpiprazole was generally well tolerated over 12 weeks in this vulnerable patient population.
Trial Registration
ClinicalTrials.gov Identifier:NCT03548584
Publisher URL: https://jamanetwork.com/journals/jamaneurology/fullarticle/2811629
DOI: 10.1001/jamaneurol.2023.3810
Keeping up-to-date with research can feel impossible, with papers being published faster than you'll ever be able to read them. That's where Researcher comes in: we're simplifying discovery and making important discussions happen. With over 19,000 sources, including peer-reviewed journals, preprints, blogs, universities, podcasts and Live events across 10 research areas, you'll never miss what's important to you. It's like social media, but better. Oh, and we should mention - it's free.
Researcher displays publicly available abstracts and doesn’t host any full article content. If the content is open access, we will direct clicks from the abstracts to the publisher website and display the PDF copy on our platform. Clicks to view the full text will be directed to the publisher website, where only users with subscriptions or access through their institution are able to view the full article.


